Entamoeba histolytica
Entamoeba histolytica is an invasive, pathogenic protozoan,
causing amoebiasis, and an important cause of diarrhea in developing countries.
Our understanding of its epidemiology has dramatically changed since this
amoeba was distinguished from another morphologically similar one, Entamoeba dispar, a non pathogenic and commensal parasite. These two species
can now be distinguished mainly through molecular and immunological
proceduresis an invasive, pathogenic protozoan,
causing amoebiasis, and an important cause of diarrhea in developing countries.
Our understanding of its epidemiology has dramatically changed since this
amoeba was distinguished from another morphologically similar one, Entamoeba dispar, a non pathogenic and commensal parasite. These two species
can now be distinguished mainly through molecular and immunological
procedures
The life cycle of the parasite is represented by two forms: the cyst and the trophozoite. The cyst is the infective and non motile form of the parasite. It is excreted in the feces and can survive for weeks in the environment. Mature cysts possess 4 nuclei and average 20 µm in diameter. The trophozoite is the motile form, with a size ranging from 10 to 60 µm. It colonizes the intestinal tract leading mainly to tissue destruction and secretory bloody diarrhea.
Amoebiasis is basically an acute disease acquired by: (i) ingestion of cysts present in contaminated food, water, or plants, (ii) through person to person contact, (iii) exposure in endemic areas, and (iv) swimming in contaminated water. Clinical manifestations range from the asymptomatic carrier state to dysenteric symptoms represented by abdominal pain and bloody diarrhea.
The organism can be prevalent in cold
regions as well as tropical and subtropical regions that have contaminated
water. In fact, E. histolytica is an important cause of morbidity and/or mortality
wherever sewage facilities are inadequate. As is the case for other intestinal
protozoan pathogens, wastewater treatment techniques are reported not to be
very efficient for E. histolytica elimination possibly because of their resistance to
disinfectants and the small size of the cysts. Stabilization ponds have been
reported to be more effective than activated sludge for their abatement.
Sedimentation and filtration can enhance the removal of cysts from
wastewater.
Plasmodium
Plasmodium, a genus of
parasitic protozoans of the sporozoan subclass
Coccidia that are the causative organisms of malaria. Plasmodium, which
infects red blood cells in mammals (including humans), birds, and reptiles,
occurs worldwide, especially in tropical and temperate zones. The organism is
transmitted by the bite of the female Anopheles mosquito. Other
insects and some mites may also transmit forms of malaria to animals.
Five
species cause human malaria: P. vivax (producing the most
widespread form), P. ovale(relatively uncommon), P. falciparum (producing the
most severe symptoms), P. malariae, and P. knowlesi.
There are several species that have been isolated from chimpanzees, including P. reichenowi and P. gaboni. P. falciparum, P. gaboni, and other
species have been isolated from gorillas. Examples of parasites found in
reptiles include P. mexicanum and P. floridense, and those in birds include P. relictum and P. juxtanucleare.
Plasmodium species exhibit three life-cycle
stages—gametocytes, sporozoites,
and merozoites. Gametocytes within a mosquito
develop into sporozoites. The sporozoites are transmitted via the saliva of a
feeding mosquito to the human bloodstream. From there they enter liver parenchyma
cells, where they divide and form merozoites. The merozoites are released into
the bloodstream and infect red blood cells. Rapid division of the merozoites
results in the destruction of the red blood cells, and the newly multiplied
merozoites then infect new red blood cells. Some merozoites may develop into
gametocytes, which can be ingested by a feeding mosquito, starting the life
cycle over again. The red blood cells destroyed by the merozoites liberate
toxins that cause the periodic chill-and-fever cycles that are the typical
symptoms of malaria. P. vivax, P. ovale, and P. falciparum repeat this chill-fever cycle every
48 hours (tertian malaria), and P. malariae repeats it every 72 hours (quartan
malaria). P. knowlesi has a 24-hour life cycle and thus can
cause daily spikes in fever.
Ascaris lumbricoides
Ascaris lumbricoides is an intestinal round worm. It
is the largest intestinal nematode to infect Human. The adult worm lives in
small intestine and grow to a length of more than 30 cm. Human is only the
natural host and reservoir of infection.
The round worm infection occurs worldwide. The number of infected persons is
estimated to be more than 2 billion.
Morphology:
Adult:
The
round worm resembles to earthworm. It is elongated tapering to both end,
anterior being thinner than posterior. Freshly excreted worm is yellowish pink
in color, which gradually changes to white.
The
worm is sexually diamorphic.
§ Adult
male: 15-30 cm in length, 3-4 mm in diameter, tail curved
§ Adult
female; 20-40 cm length, 2-6mm diameter, tail straight
Egg:
Ascaris
egg is round or oval, 60*40 µm size, thick brown shell and have rough surface.
It is the infective form of parasite.
§ i)
Un fertilized egg; large, more elongated (38-55*78-105) µm
§ ii)
fertilized egg; ovoid (35-50*50-70)µm, golden brown color
Life cycle:
The
life cycle of Ascaris completes in single host. Human.
§ Adult
worm lives in small intestine
§ Stages
in life cycle:
Stage I: Eggs in faeces
§ Sexually
mature female produces as many as 200,000 eggs per day, which are shed along
with faeces in unembryonated form. They are non infective.
Stage II: Development in soil
§ Embryonation
occurs in soil as optimum temperature of 20-25C with sufficient moisture and O2
§ Infective
larva develops within egg in about 3-6 weeks.
Stage III: Human infection and liberation of larvae
§ Human
get infection with ingestion of embryonated egg contaminated food and water
§ Within
embryonated state inside egg, first stage larvae develops into second stage
larvae. This second stage larvae is known as Rhabtitiform larvae
§ Second
stage larve is stimulated to hatch out by the presence of alkaline pH in small
intestine and solubilization of its outer layer by bile.
Stage IV: migration of larvae through lungs
§ Hatched
out larvae penetrates the intestinal wall and carried to liver through portal
circulation
§ It
then travels via blood to heart and to lungs by pulmonary circulation within
4-7 days of infection.
§ The
larvae in lungs molds twice, enlarge and breaks into alveoli.
Stage V: Re-entry to stomach and small intestine
§ From
alveoli, the Larvae then pass up through bronchi and into trachea and then
swallowed.
§ The
larvae passes down the oesophagus to the stomach and reached into small
intestine once again.
§
Small intestine is the normal habitat
of Ascaris and
it colonises here.
§ Within
intestine parasite molds twice and mature into adult worm.
§ Sexual
maturation occurs with 6-10 weeks and the mature female discharges its eggs in
intestinal lumen and excreted along with faeces, continuing the life cycle.
§ The
life span of parasite is 12-18 months
Pathogenesis:
1. Mode of transmission:
§ faeco-oral route, by contaminated vegetables or water.
2. Pathogenesis:
Infection of A. lumbricoides in man is known as Ascariasis. There
are two phase in ascariasis.
Phase I: migrating larvae
§ The migrating larvae causes pathological lesions. The
severity of lesions depends upon the sensitivity of host, nutritional status of
host and number of migrating larvae.
§ During migration and molding through lungs, larvae may causes
pneumonia with low grade fever, cough and other allergic symptoms.
Phase II: Adult worm
§ Few worm in intestine produce no major symptoms and but some
time give abdominal pain especially in children.
§ The adult worm produce trauma in host tissue and the wandering
adults may block the appendical lumen or common bile duct and even small
intestine.
§ Large number of adult worms affects the nutritional status
of host by robbing the nutrition leading to malnutrition and growth retardation
in children.
§ The metabolites of living or dead worm are toxic and
immunogenic.
§ lumbricoides also produces various allergic toxin, which
manifests fever, conjunctivitis and irritation.
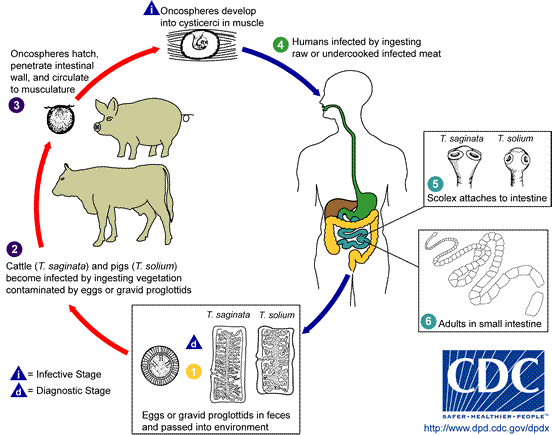
Taenia
Taenia is a flat worm that is commonly called tapeworm because of its flat and ribbon-like
body. Taenia solium is the pork tapeworm(hookworm)
and the secondary host is pig, while T. saginata is the beef tapeworm whose secondary hosts are cows,
buffalos, sheep and goat.
Adult tapeworm lives in the small intestine of man
attached to the intestinal mucosa by its scolex. The larval stage occurs in the
tissues of the secondary host which is usually pig but sometimes other animals
also.
Morphology
T. solium is long, whitish, dorsoventrally flattened
and ribbon-like worm that can reach a length of 2-3 meters. Body is divided
into hundreds of segments called proglottids.
There are three regions in the body, the scolex,
unsegmented neck and the segmented posterior region called strobila.
Scolex is the anterior end of the body; about 1mm in
diameter with 4 cup-like suckers and an anterior rounded portion called the rostellum having 20-30 curved chitinous
hooks, which are used for attachment to the intestinal wall of the host.
Neck is short, narrow and unsegmented area behind the
scolex. This is the growth zone or area of proliferation where new segments or
proglottids are added to the body.
Strobila forms the bulk of body and consists of a series of
proglottids arranged in a linear fashion. The strobila may contain as many as
800-900 proglottids. Proglottids are self-contained units, each with a complete
set of both male and female reproductive organs and a part of excretory and
nervous systems. The youngest proglottid is just behind the neck and the oldest
is at the posterior end of strobila.
 |
| Taenia solium (Pig wom) Taenia Saginata (Beef worm) |