NOTIFICATIONS
Saturday, September 30, 2017
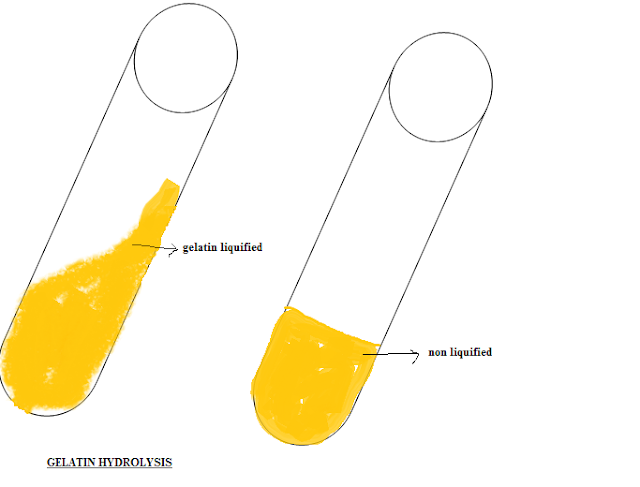
Gelatin Hydrolysis Test
Interpretation:
Friday, September 29, 2017
Catalase Test
- Glass slide/ test tube
- Pure growth of the organisms will transfer the clean slide by using inoculation loop or glass rod.
- Immediately add a drop of 3% hydrogen peroxide on bacterial culture.
- Observe the bubble formation (Effervescence).
- Take one ml of 3 % hydrogen peroxide in test tube.
Wednesday, September 27, 2017
Food Microbiology;Quality Standard : BS 5750
A British Standard for Quality Assurance (BS 5750 'Quality Systems') was published in 1979. It contained a description of the controls which it prescribed were required to be instituted in order for a supplier to claim that it was a 'Quality Assured' Organisation.
In the same way as with the registration of products to a particular standard, an organisation could not be accredited to BS 5750 unless it had been inspected (and formally accredited) by an independent authority (such as the British Standards Institute) against the standard. In contrast to the Kite Mark (which is a method of product certification), BS 5750 is a form of company certification.
The standard specifies all those 'elements' of the management system which are seen to be critical to the quality of the final product and describes how these elements are to be controlled. 3 Although its initial adoption by industry was quite slow, a number of organisations have now implemented Quality Systems commensurate with the requirements of BS 5750, although its predominance in the engineering sector remains. In fact, BS 5750 has been increasingly criticised for its continued focus on the engineering / manufacturing environments - which a quick glance at the index of the standard will show.
In more recent years, in particular, a number of non-engineering and service sector organisations have recognised that the philosophy of Quality assurance is in fact applicable to every organisation and have sought a more broadly based guideline or standard. In an effort to accommodate the views of these other industry sectors, a number of QAS (Quality Assurance Schedules) have been produced to augment / amplify the standard.
Schedule no. 8 for example, is written specifically for the Service Sector industries and contains some additional requirements and guidance on the interpretation of the standard's requirements for these organisations. In 1987 the entire standard was revised and republished and its format was significantly amended.
The text is now identical with that of its equivalent International and European Standards - ISO 9000 and EN 29000. BS 5750 is due for another major renewal in 1996 and there is currently some interesting discussion underway as to its most desirable format and scope. It should be noted that the Nuclear Industry has for some time had its own Quality System Standard. In the UK this is BS 5882 ('Specification for a Total Quality Assurance Programme for Nuclear Power Plants'), which is similar in philosophy to BS 5750.
Ref : http://mt-archive.info/Aslib-1992-Wedlake.pdf
Thursday, September 21, 2017
Oxidase Test
- To demonstrate the ability of bacteria that produces the enzyme cytochrome oxidase.
- To distinguish the bacterial groups based on oxidase activity.
(Preparation of Reagent: 1% solution of N, N, N΄, N΄ tetramethyl p-phenylene diamine dihydrochloride (5g in 5ml in distilled water). Disc preparation: Soak the sterile filter paper disc in few drops of reagents)
1. Pick the 24 hours old bacterial cultures by platinum loop/plastic loop and smear over the oxidase disc.
2. After 10 min. observe the color change on disc.
- Deep purple color change of the disc with Pseudomonas sp.,
- No color change in the disc with E.coli
- Pseudomonas sp., gives oxidase positive
- E.coli shows oxidase negative.
Tuesday, September 19, 2017
Casein Hydrolysis Test
To demonstrate the ability of certain microorganisms to produce extracellular enzymes, capable of degrading the milk protein casein.
- Bacterial culture (Bacillus sp.,)
- Skim milk agar
1. Inoculate the bacterial cultures on skim milk agar medium by streaking.
2. The plates are incubating at 37° C for 24 hrs.
3. Following incubation, observe the zone of hydrolysis around the line of growth.
- Zone of clearance around the colonies of Bacillus sp.,
- Bacillus sp., shows positive result in casein hydrolysis
Starch Hydrolysis Test
Objective:
-To determine the ability of certain microorganisms to produce extracellular enzyme amylase that breaking down starch.
-To identify the starch hydrolyzing bacteria.
1. Inoculate the bacterial cultures on starch agar medium by streaking.
2. The plates are incubating at 37° C for 24 hrs.
3. Following incubation, pour the iodine solution on medium and observe the clear zone around the area of growth.
- Clear zone around the line of growth of Bacillus sp.,
In E.coli Plate turns completely blue black color, without zone formation.
- Bacillus sp : Starch hydrolysis positive
- E. coli : Starch hydrolysis negative
Ingredients / Liter
Iodine Solution Preparation:
Monday, September 18, 2017
Food Microbiology: FPO, MPO, MMPO, HACCP, ISO 9000,14000
Fruit Product Order
Milk and Milk Products Order, 1992
Meat Food Products Order
Essential Commodities Act, 1955
The Essential Commodities Act, 1955, is a key legislation in India enacted to ensure the availability of essential commodities, control prices, prevent hoarding and black-marketing, and regulate the production, supply, and distribution of certain commodities. The Act empowers the central and state governments to regulate and control trade and commerce in essential commodities.
Historical Background
Enactment:
- The Essential Commodities Act was enacted by the Indian Parliament on 1st April 1955.
- It was introduced to address issues of scarcity and black-marketing prevalent in the post-independence period.
Amendments:
- The Act has undergone several amendments to adapt to changing economic conditions and policy requirements, including significant amendments in 2006 and 2020.
Key Provisions
Essential Commodities:
- The central government has the authority to declare any commodity as essential in the public interest.
- Examples of essential commodities include food grains, pulses, edible oils, sugar, drugs, fertilizers, and petroleum products.
Regulation of Production, Supply, and Distribution:
- The Act provides for the regulation of production, supply, and distribution of essential commodities.
- The government can impose controls and restrictions on various activities such as manufacturing, storing, transport, distribution, disposal, and consumption of essential commodities.
Control Orders:
- The government can issue control orders to regulate the prices, and stocks, and prescribe the quantities of essential commodities that can be produced, sold, or transported.
- Such orders may include directives on licensing, permitting, and stockholding limits for businesses and individuals.
Hoarding and Black-Marketing:
- The Act aims to prevent hoarding and black-marketing of essential commodities.
- Penalties for violations include fines, imprisonment, and confiscation of goods.
Price Regulation:
- The government can fix the maximum selling prices for essential commodities to ensure that they remain affordable to the public.
- This helps to stabilize prices during periods of scarcity or inflation.
Implementation Mechanisms
Central Government:
- The central government formulates policies and frameworks under the Act.
- It has the power to add or remove commodities from the list of essential commodities.
State Governments:
- State governments are responsible for implementing and enforcing the provisions of the Act within their respective states.
- They have the authority to issue control orders and take actions against violators.
Enforcement Agencies:
- Various enforcement agencies, including the police, food inspectors, and revenue officials, are involved in monitoring and ensuring compliance with the Act.
- Regular inspections, raids, and investigations are conducted to detect and prevent violations.
Key Amendments
2006 Amendment:
- Focused on improving the availability of essential commodities and preventing undue hardships to consumers.
- Introduced provisions for more stringent penalties for hoarding and black-marketing.
2020 Amendment:
- The Essential Commodities (Amendment) Act, 2020, was introduced as part of the agricultural reform bills.
- Removed cereals, pulses, oilseeds, edible oils, onions, and potatoes from the list of essential commodities, except in extraordinary situations like war, famine, extraordinary price rise, and natural calamity.
- Aimed at deregulating the agricultural sector to attract private investment and improve farm incomes.
Benefits of the Act
Consumer Protection:
- Ensures the availability of essential commodities at fair prices.
- Protects consumers from exploitation due to hoarding and black-marketing.
Price Stability:
- Helps stabilize prices of essential commodities during periods of scarcity or inflation.
- Ensures affordability and prevents sudden price spikes.
Market Regulation:
- Provides a legal framework to regulate and control the production, supply, and distribution of essential commodities.
- Promotes fair trade practices and market transparency.
Emergency Response:
- Empowers the government to take swift actions during emergencies such as natural calamities, wars, or pandemics to ensure the availability of essential commodities.
Challenges and Criticisms
Implementation Issues:
ISO 9000 Quality Management System
Benefits of ISO 9000 quality system:
Essential steps to ISO 9000:
The Role and Functions of the ISO 9000 Series in the Food Industry
The ISO 9000 series is a set of international standards for quality management systems (QMS) designed to help organizations ensure that they meet customer and regulatory requirements while continually improving their operations. In the food industry, the ISO 9000 series plays a crucial role in ensuring the quality and safety of food products, enhancing customer satisfaction, and facilitating international trade. This article explores the importance and functions of the ISO 9000 series in the food sector.
Overview of the ISO 9000 Series
The ISO 9000 series is developed and published by the International Organization for Standardization (ISO). It includes several standards, the most notable being:
- ISO 9000: Provides the fundamentals and vocabulary for QMS.
- ISO 9001: Specifies the requirements for a QMS.
- ISO 9004: Provides guidance for improving the efficiency and effectiveness of a QMS.
Role of ISO 9000 in the Food Industry
Enhancing Food Safety and Quality:
- Implementing ISO 9001 helps food companies establish robust processes and controls that ensure consistent product quality and safety. This includes thorough documentation, regular audits, and continuous monitoring of food production processes.
Compliance with Regulations:
- Food safety regulations are stringent and vary across different countries. ISO 9001 helps companies align their operations with international standards, making it easier to comply with local and global regulatory requirements.
Facilitating Trade:
- Certification to ISO 9001 can serve as a mark of quality recognized worldwide. This can help food companies enter new markets, as many international buyers and retailers prefer or require suppliers to be ISO 9001 certified.
Customer Satisfaction:
- By focusing on quality management principles such as customer focus, leadership, engagement of people, process approach, and continual improvement, ISO 9001 helps food companies enhance customer satisfaction. Meeting or exceeding customer expectations can lead to increased loyalty and repeat business.
Risk Management:
- The ISO 9001 standard emphasizes a risk-based thinking approach, helping food companies identify potential risks in their processes and implement preventive measures. This proactive approach to risk management is crucial for maintaining food safety and quality.
Functions of ISO 9000 Series in the Food Industry
Standardization of Processes:
- ISO 9001 provides a framework for standardizing food production processes, ensuring that every product meets consistent quality standards. This includes detailed procedures for sourcing raw materials, manufacturing, packaging, and distribution.
Continuous Improvement:
- One of the core principles of ISO 9001 is continual improvement. The standard encourages food companies to regularly review and improve their processes, leading to enhanced efficiency, reduced waste, and better product quality over time.
Document Control:
- Proper documentation is critical in the food industry for traceability, quality control, and regulatory compliance. ISO 9001 outlines requirements for document control, ensuring that all critical information is accurately recorded, easily accessible, and regularly updated.
Internal Audits and Reviews:
- ISO 9001 requires organizations to conduct internal audits to evaluate the effectiveness of their QMS. These audits help identify areas for improvement and ensure that processes are being followed correctly. Management reviews are also mandated to assess the overall performance of the QMS and make strategic decisions.
Supplier Management:
- Ensuring the quality of raw materials and ingredients is vital for food safety. ISO 9001 provides guidelines for supplier evaluation and management, helping food companies establish strong, reliable supply chains.
Employee Training and Competence:
- The standard emphasizes the importance of employee competence and training. By ensuring that staff are properly trained and knowledgeable about their roles, food companies can maintain high standards of quality and safety in their operations.
Conclusion
The ISO 9000 series, particularly ISO 9001, is instrumental in the food industry for maintaining and enhancing the quality and safety of food products. By providing a structured framework for quality management, it helps food companies comply with regulations, improve processes, manage risks, and satisfy customers. The implementation of ISO 9001 not only boosts operational efficiency but also opens up new opportunities in global markets, underscoring its significant role in the food sector.
ISO 14000 Quality Management Systems
Overview
The ISO 14000 family of standards is focused on environmental management systems (EMS). It helps organizations minimize their environmental impact, comply with applicable laws and regulations, and continuously improve in these areas. The most well-known standard within this series is ISO 14001, which specifies the requirements for an effective EMS.
Key Components of ISO 14000
ISO 14001: Environmental Management Systems - Requirements with Guidance for Use:
- Specifies the requirements for establishing, implementing, maintaining, and improving an EMS.
- Helps organizations improve their environmental performance through more efficient use of resources and reduction of waste.
ISO 14004: Environmental Management Systems - General Guidelines on Principles, Systems, and Support Techniques:
- Provides guidance on the establishment, implementation, maintenance, and improvement of an EMS.
- Offers a broader perspective and practical advice for organizations.
ISO 14020 Series: Environmental Labels and Declarations:
- Addresses different types of environmental labels and declarations, including eco-labeling.
- Provides principles and procedures for their development.
ISO 14030 Series: Environmental Performance Evaluation:
- Provides guidelines on environmental performance indicators.
- Helps organizations measure and communicate their environmental performance.
ISO 14040 Series: Life Cycle Assessment:
- Provides principles and framework for conducting life cycle assessments (LCAs).
- Focuses on assessing the environmental impacts of products throughout their life cycle.
Key Elements of ISO 14001
Environmental Policy:
- Organizations must establish an environmental policy that reflects their commitment to compliance, prevention of pollution, and continual improvement.
Planning:
- Identify environmental aspects and impacts.
- Establish objectives, targets, and programs to achieve these objectives.
Implementation and Operation:
- Define roles, responsibilities, and authorities.
- Ensure competence and provide training.
- Establish communication processes.
- Control documentation and operational procedures.
- Be prepared for emergencies.
Checking and Corrective Action:
- Monitor and measure key operations.
- Conduct internal audits.
- Manage nonconformities and take corrective and preventive actions.
Management Review:
- Periodically review the EMS to ensure its continuing suitability, adequacy, and effectiveness.
- Consider possible changes to the policy, objectives, and other elements of the EMS.
Benefits of ISO 14000
Improved Environmental Performance:
- Helps organizations systematically manage their environmental responsibilities, leading to better environmental outcomes.
Regulatory Compliance:
- Facilitates compliance with current and future environmental laws and regulations, reducing the risk of legal penalties.
Enhanced Reputation and Stakeholder Confidence:
- Demonstrates a commitment to environmental responsibility, which can improve relationships with customers, regulators, and the community.
Operational Efficiency:
- Identifying and managing environmental impacts can lead to more efficient resource use, reducing costs and waste.
Risk Management:
- Provides a framework for identifying and mitigating environmental risks, contributing to overall business resilience.
Market Advantage:
- Certification can provide a competitive edge in markets where customers and partners prioritize environmental responsibility.
Implementation Steps
Commitment and Policy Development:
- Obtain top management commitment and develop an environmental policy.
Planning:
- Conduct an initial environmental review.
- Identify environmental aspects and impacts.
- Set objectives and targets.
Implementation:
- Develop the EMS framework, including roles, responsibilities, and documentation.
- Provide training and awareness programs.
- Implement operational controls.
Monitoring and Review:
- Track performance against objectives and targets.
- Conduct internal audits and management reviews.
Certification:
- Engage an accredited certification body to conduct an external audit and achieve certification.
Conclusion
The ISO 14000 family of standards, especially ISO 14001, is essential for organizations aiming to improve their environmental performance systematically. By adopting these standards, organizations can ensure compliance with regulations, reduce their environmental impact, and enhance their reputation and operational efficiency. Implementing an EMS based on ISO 14001 involves a structured approach to policy development, planning, implementation, monitoring, and continuous improvement, providing substantial benefits to both the organization and the environment.
Hazard Analysis and Critical Control Point (HACCP)
HACCP (Hazard Analysis and Critical Control Points) is a systematic preventive approach to food safety that identifies, evaluates, and controls hazards that are significant for food safety. It is designed to prevent, eliminate, or reduce hazards to acceptable levels. HACCP is widely recognized and adopted in the food industry to ensure the safety of food products.
HACCP Principles
The HACCP system is based on seven core principles:
Conduct a Hazard Analysis:
- Identify potential hazards that could occur in the food production process.
- Analyze hazards to determine which ones are significant for food safety.
Determine Critical Control Points (CCPs):
- Identify points in the process where control is essential to prevent or eliminate a hazard or reduce it to an acceptable level.
Establish Critical Limits:
- Define maximum and/or minimum values (such as temperature, time, pH) to which biological, chemical, or physical parameters must be controlled at a CCP to prevent, eliminate, or reduce hazards.
Establish Monitoring Procedures:
- Develop procedures to monitor CCPs to ensure that they are within critical limits.
- Monitoring should provide real-time data to allow for immediate corrective actions if needed.
Establish Corrective Actions:
- Define actions to be taken when monitoring indicates that a CCP is not within the established critical limits.
- Corrective actions should ensure that the CCP is brought back under control and prevent potentially unsafe food from reaching consumers.
Establish Verification Procedures:
- Develop procedures to verify that the HACCP system is working effectively.
- This can include validation of the HACCP plan, CCPs, critical limits, monitoring activities, and corrective actions.
Establish Record-Keeping and Documentation Procedures:
- Maintain documentation and records that demonstrate the effective application of these principles.
- Records should include hazard analysis, CCP determination, critical limits, monitoring systems, corrective actions, verification procedures, and the HACCP plan itself.
Applications of HACCP
Food Production:
- HACCP is applied throughout the food production chain, from raw material production, procurement, and handling to manufacturing, distribution, and consumption of the finished product.
Food Processing:
- Involves controlling hazards in processing steps like cooking, pasteurization, and packaging.
Food Service and Retail:
- Ensures food safety in restaurants, cafeterias, and retail environments through controlled storage, preparation, and serving practices.
Agriculture:
- Applied in primary production to manage hazards related to environmental contaminants, pesticides, and animal health.
Import and Export:
- Used to ensure compliance with international food safety standards, facilitating global trade.
Steps to Implement HACCP
Assemble the HACCP Team:
- Form a multidisciplinary team with expertise in various aspects of the food production process.
Describe the Product:
- Provide a detailed description of the food product, including ingredients, processing methods, and packaging.
Identify Intended Use and Consumers:
- Determine the intended use of the product and the target consumer group.
Construct Flow Diagrams:
- Develop a detailed flow diagram of the entire process, from raw materials to final product.
Conduct a Hazard Analysis:
- Identify and analyze potential hazards at each step of the process, considering biological, chemical, and physical risks.
Determine CCPs:
- Identify points in the process where control is critical for safety.
Establish Critical Limits:
- Set critical limits for each CCP to ensure safety.
Establish Monitoring Procedures:
- Develop methods for monitoring CCPs to ensure they are within critical limits.
Establish Corrective Actions:
- Define actions to take when monitoring indicates a deviation from critical limits.
Establish Verification Procedures:
- Create procedures to verify that the HACCP system is functioning as intended.
- Establish Documentation and Record-Keeping:
- Maintain comprehensive records and documentation of the HACCP system and its implementation.
Benefits of HACCP
Enhanced Food Safety:
- Reduces the risk of foodborne illnesses by proactively managing potential hazards.
Regulatory Compliance:
- Helps meet national and international food safety regulations and standards.
Improved Product Quality:
- Ensures consistent quality of food products by controlling key processes.
Customer Confidence:
- Builds trust with consumers and stakeholders through demonstrated commitment to food safety.
Reduced Costs:
- Prevents costly food recalls and waste by identifying and controlling hazards early in the process.
Market Access:
- Facilitates access to markets that require HACCP certification, including many international markets.
Challenges in Implementing HACCP
Resource Intensive:
- Requires significant time, effort, and expertise to develop and maintain the system.
Training Needs:
- Employees at all levels need training to understand and effectively implement HACCP principles.
Complexity of Processes:
- Complex food production processes may make it difficult to identify all potential hazards and CCPs.
Documentation and Record-Keeping:
- Maintaining detailed records and documentation can be burdensome but is essential for verification and compliance.
Conclusion
HACCP is a vital system for ensuring food safety and quality across the food industry. By systematically identifying, evaluating, and controlling hazards, HACCP helps protect public health, ensures regulatory compliance, and enhances consumer confidence in food products. Despite the challenges in implementation, the benefits of adopting HACCP far outweigh the difficulties, making it a cornerstone of modern food safety management.
History of HACCP
The development of the Hazard Analysis and Critical Control Points (HACCP) system marks a significant milestone in food safety management. Here's a detailed look at the history and evolution of HACCP:
1959-1960s: The Origins
NASA and Pillsbury Collaboration:
- The origins of HACCP can be traced back to the late 1950s and early 1960s when the National Aeronautics and Space Administration (NASA) sought to ensure the safety of food for astronauts.
- NASA collaborated with the Pillsbury Company, the U.S. Army Natick Laboratories, and the U.S. Air Force Space Laboratory Project Group to develop a system that would produce zero-defect foods for space missions.
Concept Development:
- The traditional end-product testing methods were deemed insufficient for guaranteeing the safety and quality of food in space.
- Pillsbury, led by microbiologist Dr. Howard Bauman, developed a proactive system focusing on preventing hazards rather than detecting them after they had occurred.
1971: Introduction to the Public
National Conference on Food Protection:
- HACCP was introduced to the public at the National Conference on Food Protection in 1971.
- Dr. Howard Bauman and his team presented the HACCP concept, emphasizing its preventive approach to food safety.
FDA Adoption:
- Following its introduction, the U.S. Food and Drug Administration (FDA) began to adopt HACCP principles for low-acid canned foods, which are particularly vulnerable to Clostridium botulinum contamination.
1980s: Expansion and Endorsement
National Academy of Sciences (NAS) Report:
- In 1985, the NAS recommended that the HACCP system be used by the food industry to ensure food safety.
- The report emphasized the effectiveness of HACCP in controlling hazards in food production.
International Adoption:
- The World Health Organization (WHO) and the International Commission on Microbiological Specifications for Foods (ICMSF) endorsed HACCP.
- The Codex Alimentarius Commission, a body established by the Food and Agriculture Organization (FAO) and WHO, incorporated HACCP into its food hygiene guidelines in 1993, promoting its use globally.
1990s: Regulatory Integration
U.S. Regulatory Requirements:
- The 1990s saw the integration of HACCP into U.S. food safety regulations.
- The FDA mandated the use of HACCP for seafood (Seafood HACCP Regulation, 1995).
- The U.S. Department of Agriculture (USDA) required HACCP for meat and poultry (Pathogen Reduction/HACCP Systems Rule, 1996).
Codex Alimentarius Guidelines:
- In 1997, the Codex Alimentarius Commission published the General Principles of Food Hygiene, which included detailed HACCP guidelines. This facilitated global harmonization of food safety standards.
2000s-Present: Continued Evolution and Global Standardization
International Standards and Certification:
- HACCP principles have been integrated into various international food safety standards, including ISO 22000, which specifies requirements for a food safety management system incorporating HACCP principles.
- Global food safety initiatives, such as the Global Food Safety Initiative (GFSI), recognize HACCP-based standards like the British Retail Consortium (BRC) and the Safe Quality Food (SQF) Program.
Continuous Improvement:
- The HACCP system continues to evolve, incorporating advances in food science and technology.
- Ongoing research and development efforts aim to refine HACCP principles and improve their application across diverse food production environments.
Key Milestones in HACCP History
- 1959-1960: Development of HACCP by NASA and Pillsbury.
- 1971: Introduction of HACCP at the National Conference on Food Protection.
- 1985: NAS endorses HACCP for food safety.
- 1993: Codex Alimentarius incorporates HACCP into its food hygiene guidelines.
- 1995: FDA mandates HACCP for seafood.
- 1996: USDA mandates HACCP for meat and poultry.
- 1997: Codex Alimentarius publishes detailed HACCP guidelines.
- 2005: ISO 22000 standard, incorporating HACCP principles, is published.
Bureau of Indian Standards
Overview
The Bureau of Indian Standards (BIS) is the national standards body of India, responsible for the development and implementation of national standards, certification, and quality assurance. It operates under the Ministry of Consumer Affairs, Food and Public Distribution. BIS aims to promote and regulate standardization, marking, and quality certification of goods, ensuring their safety and reliability for consumers.
Historical Background
Establishment:
- BIS was established on April 1, 1987, under the Bureau of Indian Standards Act, 1986.
- It replaced the Indian Standards Institution (ISI), which was set up in 1947.
Legislative Framework:
- The BIS Act, 2016, provides the statutory framework for BIS, enhancing its powers to ensure better compliance and enforcement of standards.
Structure of BIS
Governing Council:
- The apex body that oversees the functioning of BIS.
- Comprises representatives from various ministries, industry associations, consumer organizations, and professional institutions.
Technical Committees:
- More than 25 divisions and 1,200 technical committees responsible for the formulation of standards across different sectors.
- Includes experts from industry, academia, research institutions, and government.
Regional and Branch Offices:
- BIS operates through a network of regional and branch offices across India to facilitate standardization activities and certification processes.
Key Functions and Responsibilities
Standardization:
- Developing Indian Standards (IS) for products, processes, systems, and services.
- Ensuring these standards align with international norms and practices.
Certification:
- Providing certification schemes like the ISI mark, which signifies conformity to Indian Standards.
- Operating various certification programs, including product certification, hallmarking for precious metals, and eco-mark for environmentally friendly products.
Quality Control and Assurance:
- Conducting inspections and testing of products to ensure they meet specified standards.
- Maintaining laboratories for the testing and analysis of products.
Consumer Protection:
- Promoting consumer awareness about standards and certification marks.
- Addressing consumer complaints and grievances related to the quality of certified products.
Training and Capacity Building:
- Offering training programs for industry professionals, quality managers, and auditors on standardization and quality assurance.
- Organizing seminars, workshops, and conferences on standardization and related topics.
International Cooperation:
- Participating in international standardization activities and aligning Indian standards with global benchmarks.
- Collaborating with international bodies like ISO (International Organization for Standardization) and IEC (International Electrotechnical Commission).
Key Initiatives and Programs
Product Certification Scheme:
- The most popular BIS certification scheme, granting the ISI mark for a wide range of products.
- Ensures that products meet the required safety, quality, and performance standards.
Compulsory Registration Scheme (CRS):
- Requires certain electronic and IT products to be registered with BIS and conform to relevant Indian Standards.
- Aims to prevent substandard and unsafe products from being sold in the market.
Hallmarking Scheme:
- A certification for the purity of precious metals like gold and silver.
- Ensures consumers get accurate information about the purity of the jewelry they purchase.
Eco-Mark Scheme:
- Certifies environmentally friendly products.
- Encourages manufacturers to produce goods with minimal environmental impact.
National Building Code of India (NBC):
- A comprehensive code for the construction industry, providing guidelines for the planning, design, and construction of buildings.
- Ensures safety, health, and sustainability in building practices.
Challenges and Future Directions
Enhancing Compliance:
- Ensuring widespread compliance with standards across diverse industries and regions.
- Increasing the frequency and coverage of inspections and audits.
Technology Integration:
- Leveraging digital technologies for efficient standardization, certification, and monitoring processes.
- Implementing online portals for easier access to standards and certification services.
Global Alignment:
- Continuously updating Indian Standards to align with international standards.
- Facilitating international trade by ensuring that Indian products meet global quality and safety requirements.
Consumer Awareness:
- Enhancing consumer awareness about the significance of standards and certification marks.
- Encouraging consumers to demand certified products for assured quality and safety.
Capacity Building:
- Expanding training and development programs to build a skilled workforce in standardization and quality assurance.
- Collaborating with educational institutions to incorporate standardization studies into academic curricula.
Conclusion
The Bureau of Indian Standards (BIS) plays a pivotal role in ensuring the quality, safety, and reliability of products and services in India. Through its comprehensive standardization and certification programs, BIS protects consumer interests and enhances the competitiveness of Indian products in the global market. As the regulatory landscape evolves, BIS continues to adapt and innovate, striving to promote a culture of quality and excellence across all sectors of the Indian economy.
Codex Alimentarius Commission
Overview
The Codex Alimentarius Commission (CAC) is an international body established to develop food standards, guidelines, and codes of practice under the Joint FAO/WHO Food Standards Program. The primary goals are to ensure consumer health protection and fair practices in the food trade.
History and Establishment
Formation:
- The CAC was established in 1963 by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO).
Purpose:
- To develop international food standards to protect consumer health.
- To ensure fair practices in the food trade.
- To promote coordination of all food standards work undertaken by international governmental and non-governmental organizations.
Structure
Membership:
- The CAC has 189 members, including 188 member countries and 1 member organization (the European Union).
Committees:
- The CAC operates through various committees, which are either commodity-specific (e.g., dairy products, fresh fruits and vegetables) or general subject committees (e.g., food hygiene, food additives, pesticide residues).
Executive Committee:
- An Executive Committee, elected by the CAC, provides direction to the Commission’s work.
Key Functions
Standard Development:
- Developing food standards, guidelines, and codes of practice to ensure the quality and safety of food products.
- These standards cover a wide range of food products and issues, including contaminants, hygiene, labeling, and pesticide residues.
Risk Assessment:
- Collaborating with scientific expert bodies like the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the Joint FAO/WHO Meeting on Pesticide Residues (JMPR) to conduct risk assessments.
- Using scientific data to set maximum limits for food additives, contaminants, and pesticide residues.
Consumer Protection:
- Ensuring that the food products are safe, of good quality, and suitable for consumption.
- Providing guidelines for nutritional labeling and health claims to prevent misleading information.
Trade Facilitation:
- Harmonizing food standards to facilitate international trade by ensuring that food products meet globally recognized safety and quality criteria.
- Working to eliminate trade barriers and disputes related to food safety and quality.
Capacity Building:
- Assisting developing countries in building their capacity to produce and regulate food products that meet international standards.
- Providing training and technical assistance to improve food safety and quality management systems.
Notable Achievements
Codex Alimentarius (Food Code):
- The CAC has developed a comprehensive collection of standards, guidelines, and codes of practice known as the Codex Alimentarius or the "Food Code."
- This includes standards for food safety, food hygiene, food labeling, and food additives.
Harmonization of Standards:
- Promoting harmonization of food standards internationally, which has been pivotal in reducing trade barriers and ensuring consistent food safety practices worldwide.
Global Recognition:
- Codex standards are recognized by the World Trade Organization (WTO) as the benchmark for international food safety requirements, thereby influencing national food regulations and trade agreements.
Current Focus and Challenges
Emerging Food Safety Issues:
- Addressing new and emerging food safety issues such as antimicrobial resistance, food fraud, and the impact of climate change on food safety.
Inclusivity and Participation:
- Enhancing the participation of developing countries in the standard-setting process to ensure that Codex standards reflect global needs and contexts.
Consumer Awareness:
- Raising awareness about food safety and the importance of Codex standards among consumers, food producers, and regulators.
Technological Advances:
- Incorporating advancements in food science and technology into standards and guidelines to keep pace with innovations in food production and processing.
Conclusion
The Codex Alimentarius Commission plays a crucial role in ensuring food safety and facilitating international food trade. By developing science-based food standards and promoting their global harmonization, the CAC helps protect consumer health and ensure fair trade practices. Its ongoing efforts to address emerging food safety challenges and to involve all member countries in the standard-setting process underscore its commitment to maintaining the safety and integrity of the global food supply.
FSSAI ( Food Safety and Standard Authority of India)
The Food Safety and Standards Authority of India (FSSAI) is the apex regulatory body established to ensure food safety and standards in India. Formed under the Food Safety and Standards Act, 2006, FSSAI operates under the Ministry of Health & Family Welfare, Government of India. Its primary role is to protect and promote public health through the regulation and supervision of food safety.
Historical Background
Before the establishment of FSSAI, multiple laws and regulatory agencies governed food safety in India. This fragmented approach led to inefficiencies and inconsistencies. To address these issues, the Food Safety and Standards Act, 2006, was enacted, consolidating various laws and creating a single regulatory authority—FSSAI. The organization officially began its operations in 2011.
Structure of FSSAI
Central Advisory Committee:
- Provides recommendations on policy and strategic matters.
- Includes representatives from central and state governments, food industry, consumer organizations, and academic institutions.
Scientific Panels and Committees:
- Consist of experts in various fields such as food additives, contaminants, pesticides, and nutrition.
- Responsible for risk assessment and setting safety standards based on scientific evidence.
Regional Offices and State Food Authorities:
- Ensure the implementation of food safety regulations at the state and local levels.
- Conduct inspections, enforcement, and compliance activities.
Key Functions and Responsibilities
Regulation and Standard Setting:
- Develops and updates food safety standards based on scientific risk assessments.
- Issues regulations on food additives, contaminants, pesticide residues, labeling, and food products.
Licensing and Registration:
- Mandates that all food business operators (FBOs) obtain licenses or registrations to ensure they meet safety standards.
- Categorizes FBOs based on the size and nature of their operations.
Inspection and Compliance:
- Conducts regular inspections of food establishments to ensure compliance with food safety regulations.
- Takes enforcement actions against non-compliant FBOs, including penalties and closure of operations if necessary.
Risk Assessment and Management:
- Performs risk assessments to identify potential food safety hazards.
- Develops strategies and measures to mitigate identified risks.
Surveillance and Monitoring:
- Implements food safety surveillance programs to monitor the safety of food products in the market.
- Collects and analyzes data on foodborne illnesses and contamination incidents.
Consumer Awareness and Education:
- Conducts public awareness campaigns on food safety and hygiene practices.
- Provides educational resources and tools to help consumers make informed choices about food.
Coordination and Collaboration:
- Collaborates with international food safety organizations, such as the Codex Alimentarius Commission, to align with global standards.
- Works with other government agencies, industry stakeholders, and consumer groups to enhance food safety measures.
Key Initiatives and Programs
Safe and Nutritious Food (SNF):
- A nationwide campaign aimed at promoting safe and nutritious food at home, schools, workplaces, and eateries.
- Includes initiatives like Eat Right India, which encourages healthy eating habits.
Food Safety Training and Certification (FoSTaC):
- Provides training programs for food handlers and FBOs to improve their knowledge and practices related to food safety.
Food Fortification:
- Promotes the fortification of staple foods like rice, wheat flour, milk, and edible oil with essential nutrients to address micronutrient deficiencies.
Clean Street Food Hubs:
- Aims to upgrade and ensure the safety of street food vendors through training, certification, and infrastructure improvements.
Eat Right Movement:
- Encourages consumers to adopt a healthy, balanced diet and make informed food choices.
- Involves various stakeholders, including food companies, health professionals, and consumer organizations.
Challenges and Future Directions
Ensuring Compliance:
- Despite the regulations, ensuring compliance among millions of small-scale food businesses remains a significant challenge.
- FSSAI is working on strengthening inspection mechanisms and increasing the frequency of checks.
Capacity Building:
- Continuous training and capacity building for food inspectors, laboratory personnel, and FBOs are essential for effective implementation of food safety regulations.
Technology Integration:
- Leveraging technology for better surveillance, traceability, and transparency in the food supply chain is a focus area.
- Initiatives like the Food Safety Compliance System (FoSCoS) aim to streamline licensing, inspection, and compliance processes.
Public-Private Partnerships:
- Enhancing collaboration with private sector stakeholders to improve food safety standards and practices.
- Encouraging industry self-regulation and corporate responsibility in ensuring food safety.
Conclusion
FSSAI plays a crucial role in safeguarding public health by ensuring the safety and quality of food products in India. Through its comprehensive regulatory framework, scientific approach, and collaborative efforts, FSSAI aims to build a robust food safety system. As the food industry evolves, FSSAI continues to adapt and innovate, striving to protect consumers and promote a culture of food safety across the nation.
ISO 22000 series
Overview
The ISO 22000 series is a set of international standards focusing on food safety management systems (FSMS). It provides a framework to ensure the safety of food throughout the entire food chain, from farm to fork. The primary standard, ISO 22000:2018, integrates principles from the Hazard Analysis and Critical Control Points (HACCP) system and other critical elements of food safety management.
Key Standards in the ISO 22000 Series
ISO 22000:2018:
- Title: "Food safety management systems — Requirements for any organization in the food chain."
- Focuses on setting up a robust FSMS that includes interactive communication, system management, prerequisite programs, and HACCP principles.
- Applicable to all organizations in the food chain, regardless of size and complexity.
ISO/TS 22002-1:2009:
- Title: "Prerequisite programs on food safety — Part 1: Food manufacturing."
- Specifies requirements for establishing, implementing, and maintaining prerequisite programs to assist in controlling food safety hazards in food manufacturing.
ISO/TS 22002-2:2013:
- Title: "Prerequisite programs on food safety — Part 2: Catering."
- Provides guidance for prerequisite programs applicable to catering services.
ISO/TS 22002-3:2011:
- Title: "Prerequisite programs on food safety — Part 3: Farming."
- Details requirements for establishing prerequisite programs in farming operations to control food safety hazards.
ISO/TS 22002-4:2013:
- Title: "Prerequisite programs on food safety — Part 4: Food packaging manufacturing."
- Specifies requirements for food safety in the manufacturing of food packaging materials.
ISO/TS 22002-5:2019:
- Title: "Prerequisite programs on food safety — Part 5: Transport and storage."
- Focuses on the control of food safety hazards during the transportation and storage of food products.
ISO 22004:2014:
- Title: "Food safety management systems — Guidance on the application of ISO 22000."
- Provides guidelines to help organizations understand and implement the requirements of ISO 22000.
ISO 22005:2007:
- Title: "Traceability in the feed and food chain — General principles and basic requirements for system design and implementation."
- Focuses on establishing a traceability system to ensure the identification of food products through all stages of production, processing, and distribution.
Key Components of ISO 22000:2018
Management Commitment and Leadership:
- Emphasizes the role of top management in establishing, implementing, and maintaining an effective FSMS.
- Requires clear communication and allocation of responsibilities within the organization.
Risk-Based Thinking:
- Incorporates risk management principles to identify, evaluate, and control food safety hazards.
- Focuses on preventive measures to mitigate risks before they impact food safety.
HACCP Principles:
- Integrates the seven principles of HACCP: conducting hazard analysis, determining critical control points, establishing critical limits, monitoring procedures, corrective actions, verification procedures, and documentation.
Interactive Communication:
- Highlights the importance of communication within the organization and with external stakeholders, including suppliers, customers, and regulatory authorities.
- Ensures transparency and clarity in communicating food safety requirements and expectations.
System Management:
- Emphasizes the need for a systematic approach to managing food safety, incorporating elements such as policy development, objective setting, planning, and continual improvement.
Prerequisite Programs (PRPs):
- Establishes basic conditions and activities necessary to maintain a hygienic environment throughout the food chain.
- Examples of PRPs include cleaning and sanitation, pest control, supplier management, and personal hygiene.
Operational Prerequisite Programs (OPRPs):
- Defines specific control measures that are essential to prevent food safety hazards that cannot be managed solely by PRPs.
Documentation and Records:
- Requires comprehensive documentation to support the implementation and maintenance of the FSMS.
- Ensures traceability, accountability, and verification of food safety practices.
Benefits of Implementing ISO 22000
Enhanced Food Safety:
- Provides a structured approach to identifying and controlling food safety hazards, reducing the risk of contamination and foodborne illnesses.
Compliance with Regulatory Requirements:
- Helps organizations meet national and international food safety regulations and standards, facilitating market access and trade.
Improved Customer Confidence:
- Demonstrates a commitment to food safety, enhancing consumer trust and brand reputation.
Operational Efficiency:
- Streamlines processes and procedures, leading to improved resource management and reduced waste.
International Recognition:
- As an internationally recognized standard, ISO 22000 certification can enhance global competitiveness and marketability.
Continual Improvement:
- Encourages ongoing evaluation and improvement of the FSMS, ensuring it remains effective and up-to-date with evolving food safety challenges.